Our Pipeline
The information contained here is under clinical investigation. Neither any product nor the technology have been approved for any use or marketing by regulatory authorities in any country.
Using our innovative technology platform, Viaskin™, we are committed to developing a pipeline of food allergy product candidates that could potentially transform the care of food-allergic patients.
Thank you to all of the patients, caregivers, collaborating physicians and research staff who have enabled our clinical trial development and helped us to advance the potential of Viaskin.
Development Pipeline
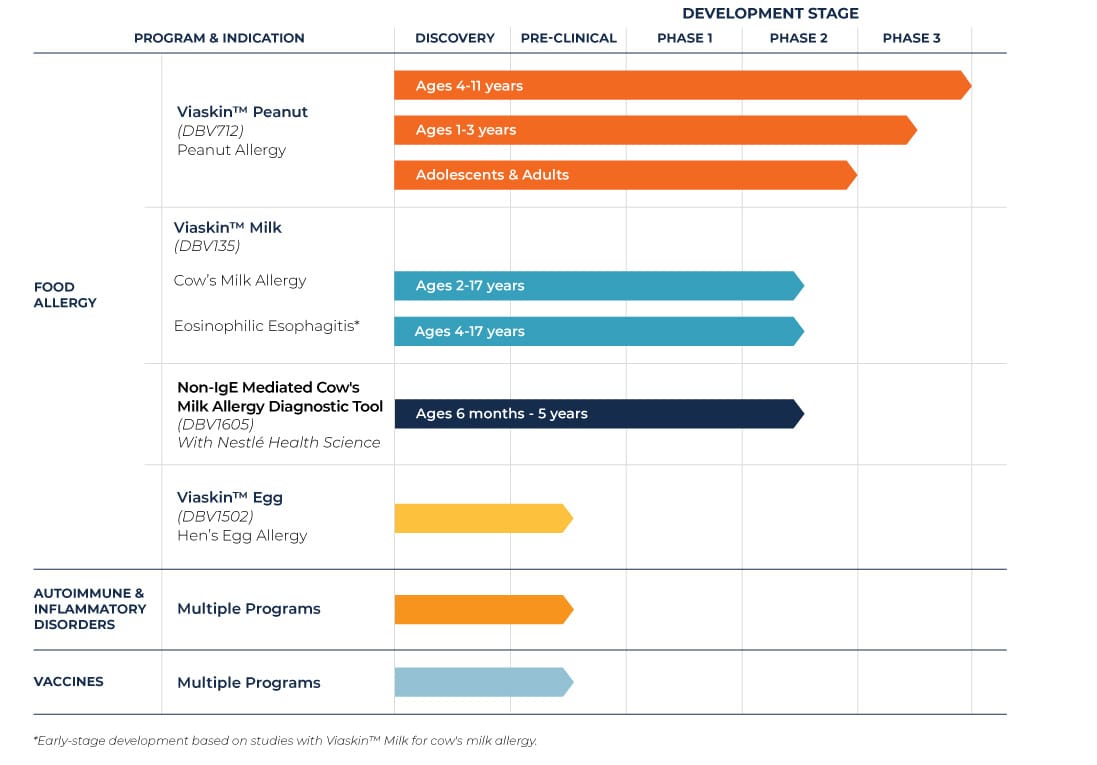
Food Allergy Clinical Trials
Viaskin™ Peanut Clinical Trials
Peanut allergy is one of the most common food allergies and can cause severe, potentially fatal, allergic reactions, as well as anaphylaxis.
We have a comprehensive clinical research program in place for Viaskin Peanut. One Phase 3 long-term study in children ages four to 11 is ongoing, as well as two Phase 3 trials in patients ages one to three.
* Viaskin Peanut is an investigational product and has not been approved for any use in any country.
Viaskin™ Milk Clinical Trials
Cow’s Milk Protein Allergy (CMPA) mainly appears in the first year of life and affects approximately 2-3 percent of the population in developed countries.
We completed a Phase 1/2 trial to study the safety and efficacy of Viaskin Milk in pediatric and adolescent patients.
Viaskin™ Egg Pre-Clinical Studies
Hen’s egg allergy is one of the most common food allergies in children. Several global studies suggest that egg allergy affects 1.5–3 percent of young children.
The third food allergy to be investigated for treatment with Viaskin is hen’s egg allergy. Pre-clinical work has been initiated for Viaskin Egg.
Other Early Stage Development Programs
Investigations Beyond Food Allergies
DBV is investigating other areas of significant unmet medical need, including eosinophilic esophagitis (EoE), inflammatory conditions and autoimmune diseases, as well as the potential application of Viaskin technology to vaccines.
Diagnostics
As we focus on diversifying our pipeline, we are also exploring the use of our technology platform in the development of diagnostic tools for food allergies. In 2016, DBV entered an exclusive global collaboration with Nestlé Health Science to develop MAG1C, a ready-to-use and standardized atopy patch test tool for the potential diagnosis of cow’s milk protein allergy in infants and toddlers.
