Development Program:
Viaskin™ Milk
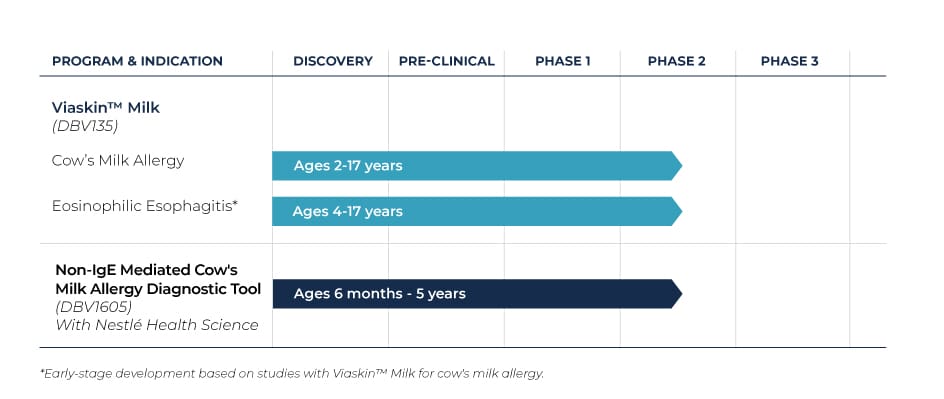
Cow’s Milk Protein Allergy (CMPA) is often the first allergy that appears during early childhood and affects approximately 2-3 percent of the population in developed countries.
We have completed a Phase 1/2 trial to study the safety and efficacy of Viaskin Milk in pediatric and adolescent patients.
Clinical Trials (IgE-mediated Cow’s Milk Allergy)
MILES (Viaskin Milk Efficacy and Safety)
MILES is a multi-center, double-blind, placebo-controlled, randomized Phase 1/2 trial to study the safety and efficacy of Viaskin Milk conducted at 17 sites in North America. The study was divided into two consecutive parts. Part A of the MILES trial was completed with no safety concerns. Part B was designed to determine a safe and effective dose in two age groups: children ages 2 to 11 and adolescents ages 12 to 17 with IgE-mediated cow’s milk protein allergy, or CMPA.
External Collaborations
Pilot Clinical Trial of Epicutaneous Immunotherapy in Cow’s Milk Allergy with Assistance Publique Hopitaux de Paris (AP-HP)
In 2006 a double-blind, placebo-controlled pilot clinical trial was initiated with AP-HP to evaluate the safety of Viaskin Milk in children. This pilot trial enrolled 21 infants (starting at three months) and children (up to age 15) with cow’s milk allergy.
Learn more
Clinical Trials (Non-IgE-mediated Cow’s Milk Allergy Diagnosis)
APTITUDE
APTITUDE is a non-randomized, triple-blinded, placebo-controlled Phase 2 trial in 27 sites in North America and Europe. This two-arm study is designed to assess the sensitivity, specificity and positive and negative predictive value of DBV1605 as a ready-to-use atopy patch test for the diagnosis of non-IgE mediated cow’s milk allergy in children ages 1 month to 5 years. This is an ongoing trial, with estimated enrollment of 230 subjects.
Learn more
* Viaskin Milk is an investigational product and has not been approved for any use in any country.
