Development Program:
Viaskin™ Peanut
Peanut allergy is one of the most common food allergies and can cause severe, potentially fatal, allergic reactions, as well as anaphylaxis.
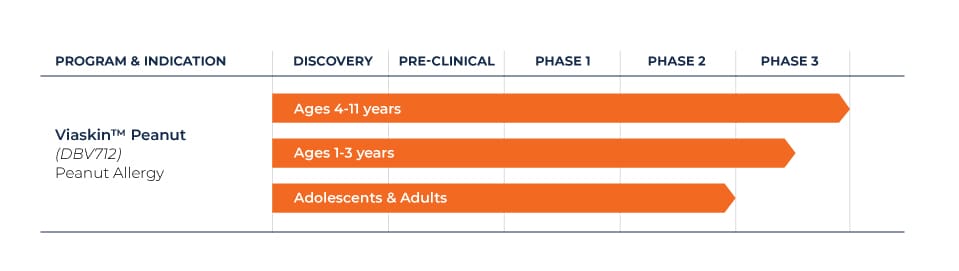
We have a comprehensive clinical research program in place for Viaskin Peanut. One Phase 3 long-term study in children ages 4 to 11 is ongoing, as well as a Phase 3 trial in patients one to three years of age.
Current Clinical Trials
VITESSE
This study is evaluating an investigational drug patch called DBV712 for 4 to 7 year old children with peanut allergy. The main purpose of this study is to learn how well the study drug patch (DBV712) works and how safe it is compared with a placebo patch in children with peanut allergy. The small amount of peanut protein in the study drug patch is designed to potentially desensitise (or make less sensitive) a peanut-allergic person by repeated exposures to very small amounts of peanut via the skin.
Learn more
Completed Clinical Trials
EPITOPE (EPIT™ in Toddlers with Peanut Allergy)
EPITOPE is a global, Phase 3 clinical trial assessing the safety and efficacy of Viaskin Peanut for the treatment of peanut-allergic patients one to three years of age. EPITOPE is a two-part, pivotal, double-blind, placebo-controlled trial.
Learn more
REALISE (Real Life Use and Safety of EPIT)
REALISE is a multicenter, randomized, double-blind, placebo-controlled Phase 3 study designed to assess the use of Viaskin Peanut 250 µg in routine medical practice and generate safety data after six months of blinded treatment, followed by up to 36 months of open-label treatment. The study enrolled 393 patients 4 to 11 years of age.
PEOPLE (PEPITES Open Label Extension Study)
Learn more
PEPITES (Peanut EPIT Efficacy and Safety Study)
Learn more
OLFUS-VIPES (Open-Label Follow-Up Study-Viaskin Peanut’s Efficacy and Safety)
Learn more
VIPES (Viaskin™ Peanut’s Efficacy and Safety)
Learn more
Phase Ib Clinical Trial
Learn more
External Collaborations: Viaskin Peanut Trials
CoFAR6 (Consortium for Food Allergy Research 6)
Learn more
ARACHILD
Learn more
* Viaskin Peanut is an investigational product and has not been approved for any use in any country.
